Whole-exome and whole-genome sequencing of 1,064 individuals with type 1 diabetes reveals novel genes for diabetic kidney disease

Type 1 diabetes (T1D) affects millions of people worldwide, and despite modern insulin treatments, about 33% of individuals develop long-term complications affecting kidneys, eyes and the cardiovascular system. Diabetic kidney disease (DKD) is the leading cause of end-stage kidney disease in Western countries, forcing individuals to depend on dialysis or kidney transplants to survive. Even in its early stages, DKD triples a person's risk of heart attacks and strokes. However, it remains unclear why some people with T1D develop serious DKD while others do not. The Finnish Diabetic Nephropathy Study (FinnDiane) aims to identify risk factors for diabetic complications with the ultimate aim to prevent diabetic complications and improve the quality of life of people with diabetes.
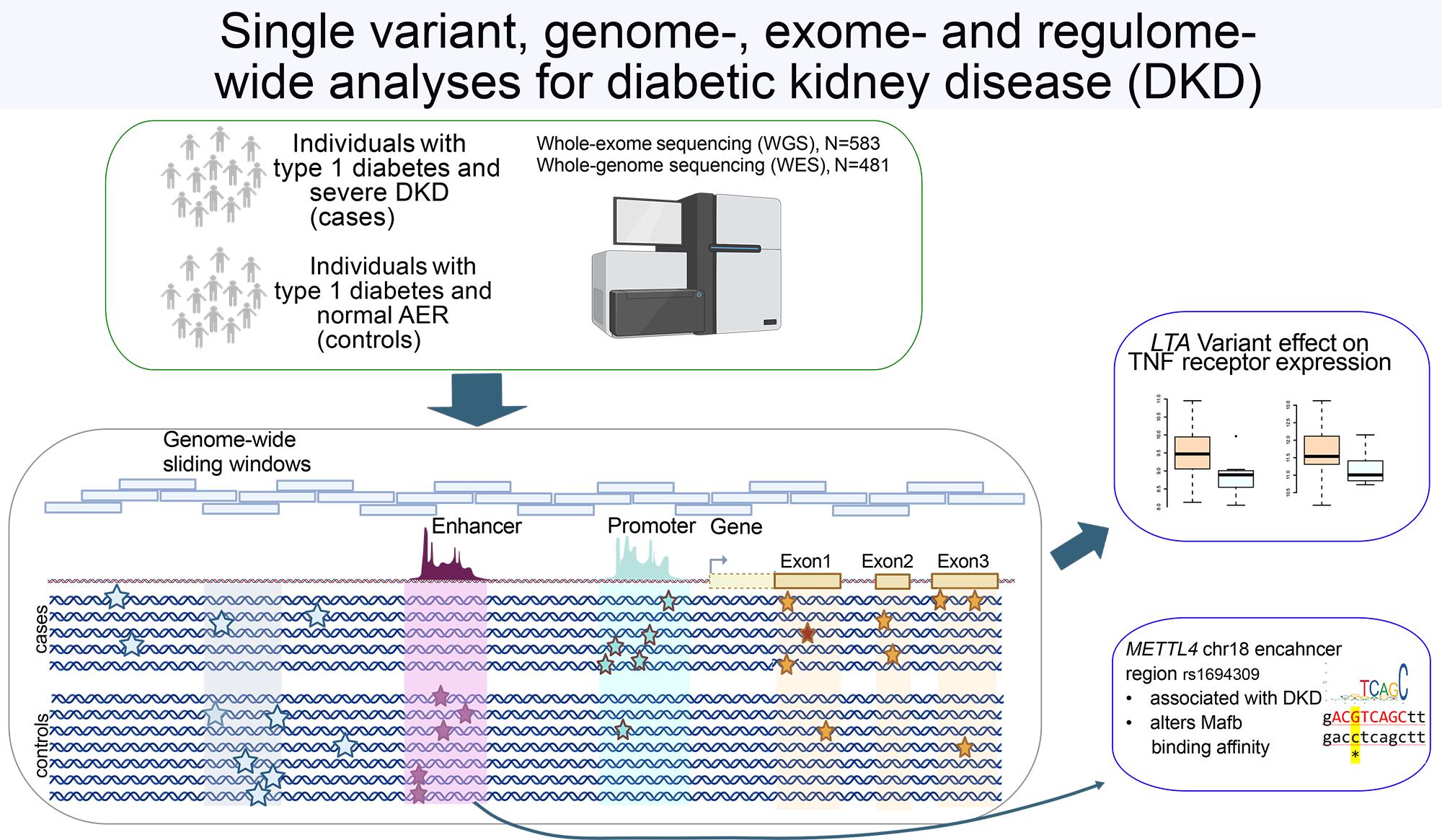
In our genetic sequencing study of over 1,000 FinnDiane study participants with T1D, we uncovered clues that might help understand why some of the individuals develop DKD. By analysing the complete genetic code of these individuals, we identified several genes and genetic variants that appear to influence DKD risk. One key discovery involves a gene LTA, which controls inflammation. People carrying a protective variant of this gene had lower levels of inflammatory proteins in their blood and were less likely to develop kidney problems. We also found a genetic switch near a METTL4 gene, which appears to work differently in men and women, potentially helping explain why men with diabetes often face a higher risk of DKD. Another discovery involves the NAT16 gene, which shows unusually high activity in certain other kidney disease.
These findings provide valuable insights into how DKD develops. Although more research is needed, understanding these genetic factors could help doctors identify high-risk individuals who need more aggressive prevention strategies. These insights from one of the largest genetic studies of DKD to date bring hope for better prevention and treatment strategies in the future.
Original article:
Whole-exome and whole-genome sequencing of 1064 individuals with type 1 diabetes reveals novel genes for diabetic kidney disease.
Haukka JK, Antikainen AA, Valo E, Syreeni A, Dahlström EH, Lin BM, Franceschini N, Krolewski AS, Harjutsalo V, Groop PH, Sandholm N; FinnDiane Study Group. Diabetologia. 2024.
Highlights 2024

A retrospective longitudinal study of 52 Finnish patients with X-linked retinoschisis
Understanding X-Linked Retinoschisis (XLRS) in Finland.

Inferring disease course from differential exon usage in the wide titinopathy spectrum
Improving prognosis prediction in titinopathies: a step toward personalized medicine.

International canine gene research database accelerates biomedical research

Burden of oral diseases predicted the development of excess weight in early adolescence

The brain insulin receptor gene network and associations with frailty index